Radioactive elements (radionuclides or radioactive isotopes) produce high energy particles and are used in a huge range of applications. Most people know about nuclear power, which converts the energy of radiation from uranium-238 or plutonium-239 into heat and then electrical power, even in small-scale form for remote applications (e.g. spacecraft). There are far more widespread uses all around us though.
Radionuclides are used in many medicinal applications. They can be used as tracers to follow fluid flow inside the body by detecting the radionuclide emitted radiation (e.g. technetium-99, thallium-201, iodine-131 and sodium-24). Medical imaging can use radioactive elements that naturally collect in particular parts of the body and image the radioactive emission. For example, iodine-131 is used to image the thyroid and other isotopes can be used for other organs, such as bones, heart, liver and lungs. Larger doses of the radionuclides (e.g. cobalt-60) are used to create a targeted radiotherapy treatment of cancer in these organs. It is even possible to detect the presence of Heliobacter pylori (an unwanted bacterium that can be in stomachs) with a simple breath test that uses carbon-14.
You may have radioactive materials in your home, school or workplace. Smoke detectors use alpha radiation from americium-241 to ionise smoke particles for detection. Glow-in-the-dark inks on clocks, watches and emergency signs that convert radioactive particle energy from promethium-147 into light.
You may also have food that has been treated with radiation. Many foods (including tomatoes, mushrooms, berries, cereals, eggs, fish and some meat products) are irradiated with gamma rays from cobalt-60 to kill micro-organisms and improve the food’s shelf life (without making the food radioactive!). Similarly, gamma radiation from caesium-137 is used to sterilise medical products such as syringes, heart valves, surgical instruments and contact lens solutions.
Radioactive elements are used in industry too. For example, the absorption of different types of radiation mean it can be used to monitor the thickness of manufactured components and sheets. Radionuclides are also used for detecting leaks from pipes, the direction of underground pipes and waste dispersal in the environment. Radioactive sources are also used in industrial imaging, with the sample placed between the radiation source and a detector. Certain isotopes are used as chemicals in order to trace chemical reaction routes, e.g. carbon-14 in photosynthesis. Similar approaches are used in biology to test when proteins undergo important ‘phosphorylation’ reactions (using phosphorus-32) to learn when their function is activated by other proteins or small chemicals.
Radioactive elements can also be used for historical dating of objects, e.g. carbon-14 dating for estimating the age of organic matter and uranium-238 for rocks. Similarly, radioactive decay from vintage drinks such as wine can be used to prove their age, since radionuclides were released into the atmosphere by nuclear explosion tests after World War II and are present in all food and drink produced since then.
With so many uses, it’s no wonder that radioactive decay is an important aspect of science and engineering!
Use this experiment to find out more!
Download the file below for the quick guide for the Radioactivity experiment (requires login) or follow these brief instructions:
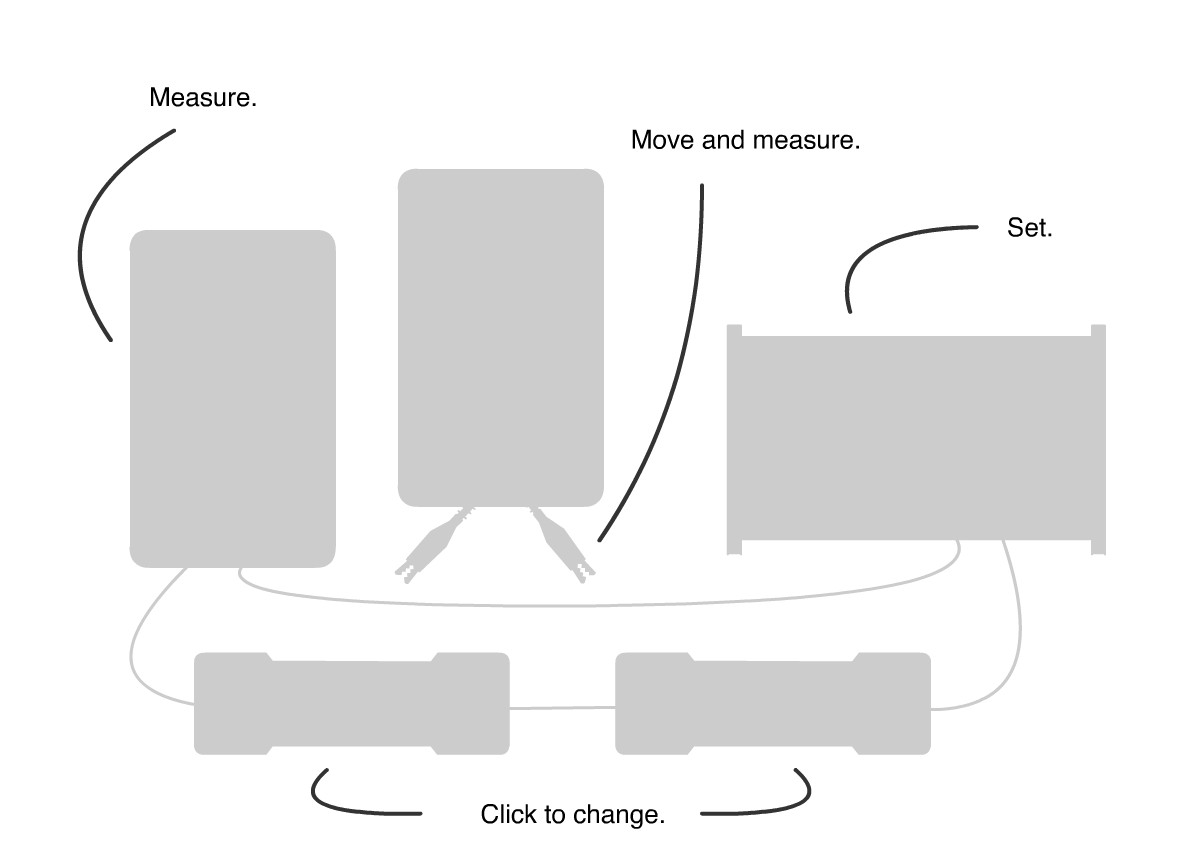
Select the radiation source:
- Click on the Source holder.
- Click on one of the radiation sources in the tray and then in the holder.
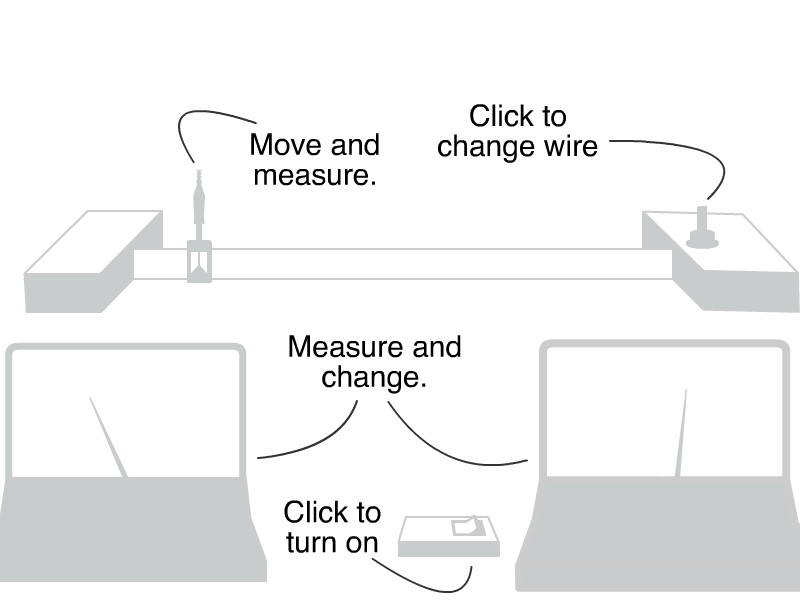
Detecting radiation:
- Click the power switch on the Geiger-Müller counter.
- Read the needle position on the dial to measure level of radiation.
Changing the filter material and thickness:
- Click on the filter holder.
- Click on the material of choice to increase its thickness in the holder by 1 mm.
- Click on the material in the holder to reduce its thickness by 1 mm.
- Measure the filter thickness using the markings inside the holder.
Change the source-detector separation:
- Click and drag the holder mount to move it along the ruler.
- Measure the mount’s position using the magnified ruler view seen while clicked on the holder mount.
Time travel!
- Move time forward by one minute, hour, day, month or year by clicking on the appropriate value on the clock.
Download the file below for activities for the Radioactivity experiment (requires login).
- ACTIVITY 1: Radioactive half-life… and time travel! (used in GCSE Physics)
- ACTIVITY 2: Inverse square law (used in A-level Physics)
- ACTIVITY 3: Which materials absorb different types of radiation?
- ACTIVITY 4: Radiation absorption strength
- ACTIVITY 5: Alpha radiation (advanced experiment)
- ACTIVITY 6: Mixed radiation sources
(Available as separate downloads or all activities)
*NEW* Now also available in editable Microsoft Word format
Or see if you can do some of the following:
- Measure the half-life of different sources (GCSE)
- Measure how detected gamma radiation varies with source-detector separation
- Find out how alpha, beta and gamma radiation is absorbed by various materials
- Explore multi-decay type radioactive sources